Liste
Précédente Suivante
Publication 66
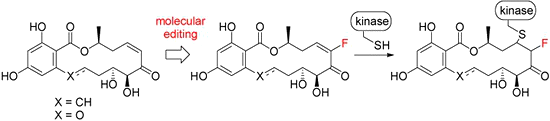
- Molecular Editing of Kinase-Targeting Resorcylic Acid Lactones (RAL): Fluoroenone RAL
R. Jogireddy, S. Barluenga, N. Winssinger
ChemMedChem 2010, 5, 670-673

Molecular editing: The resorcylic acid lactones (RAL) are known small-molecule irreversible inhibitors of select kinases, and represent a unique pharmacophore with potential for further development in kinase research. The basic pharmacophore was "edited" to improve the properties and to diversify the scaffold. Two fluoroenones were synthesized, and their preliminary biological evaluation revealed interesting activity.
DOI : 10.1002/cmdc.201000047
archive ouverte unige:24705
