Sustained rhoptry docking and discharge requires Toxoplasma gondii intraconoidal microtubule-associated proteins
SUMMARY
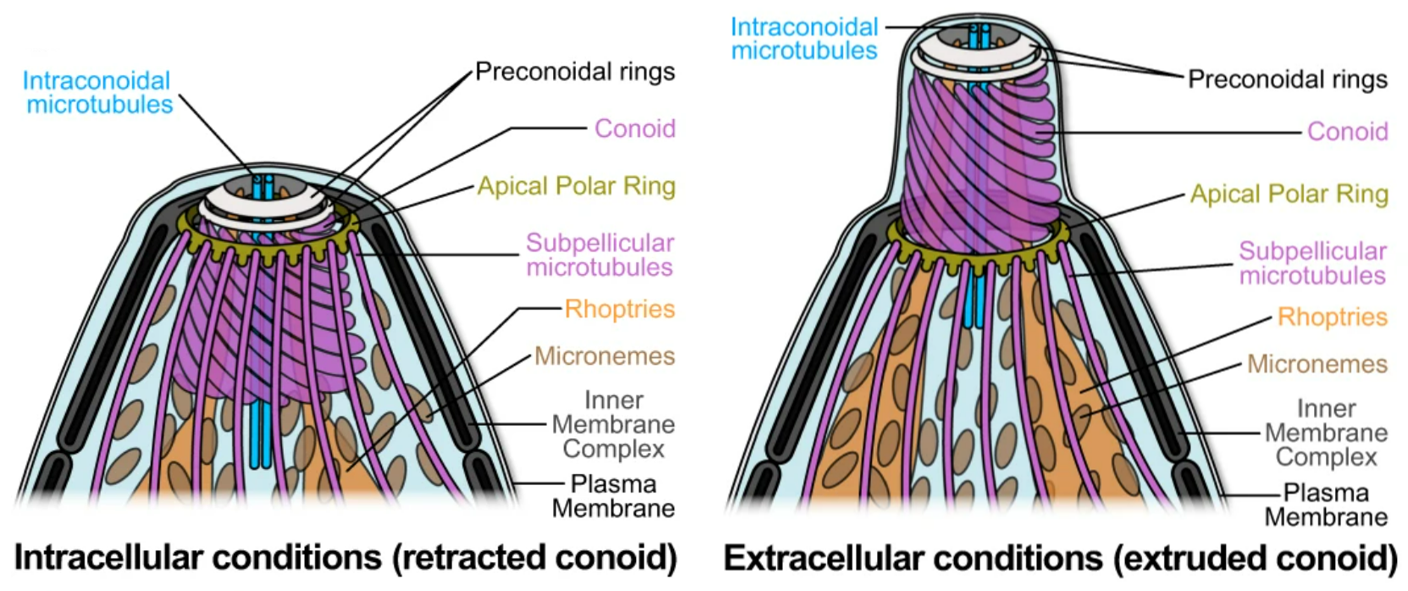
In Apicomplexa, rhoptry discharge is essential for invasion and involves an apical vesicle (AV) docking one or two rhoptries to a macromolecular secretory apparatus. Toxoplasma gondii is armed with 10-12 rhoptries and 5-6 microtubule-associated vesicles (MVs) presumably for iterative rhoptry discharge. Here, the authors led by GCIR Porf. Dominique Soldati-Favre, have addressed the localization and functional significance of two intraconoidal microtubule (ICMT)-associated proteins instrumental for invasion. Mechanistically, depletion of ICMAP2 leads to a dissociation of the ICMTs, their detachment from the conoid and dispersion of MVs and rhoptries. ICMAP3 exists in two isoforms that contribute to the control of the ICMTs length and the docking of the two rhoptries at the AV, respectively. This study illuminates the central role ICMTs play in scaffolding the discharge of multiple rhoptries. This process is instrumental for virulence in the mouse model of infection and in addition promotes sterile protection against T. gondii via the release of key effectors inducing immunity.
Full article: https://www.nature.com/articles/s41467-023-44631-y
Funding: This work was supported by the Swiss National Foundation and by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program agreement; by a David and Lucile Packard Fellowship for Science and Engineering, a Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease Program Award and a Pennsylvania Department of Health FY19 Health Research Formula Fund; by a Martin and Pamela Winter Infectious Disease Fellowship; and by the National Institutes of Health.
Why is it important?
Toxoplasma gondii is an opportunistic pathogen of medical significance, particularly in immunocompromised patients and through congenital transmission. This eukaryotic parasite can infect most warm-blooded animal cells by utilizing its apical complex, composed of cytoskeletal elements and secretory organelles. Amongst them, the rhoptries play a crucial role in invasion and subversion of the host cellular functions. T. gondii possesses 10-12 rhoptries that can be injected into non-infected host cells, presumably to induce a favorable environment for the establishment of parasitism. Additionally, two short intraconoidal microtubules (ICMTs) are closely associated with two apically docked rhoptries, potentially contributing to their positioning and discharge. Using a combination of genome editing and high-resolution imaging techniques, the authors illustrate the essential role of the ICMTs in sequential rhoptry discharge. This process is critical for establishing the infection and eliciting an immune response against the parasite, uncovering a novel aspect of host-pathogen interaction in apicomplexans.
18 Jan 2024