Labidi-Galy Intidhar

Dr. Intidhar Labidi-Galy
Pathogenesis and precision medicine in breast and ovarian cancer, Lab Leader
CMU - 6th floor, Room A06.2910.c
E-mail
Research overview

In the last decade, large-scale next-generation sequencing of DNA and RNA allowed for a new comprehension of cancer, “the emperor of all maladies” at unprecedented scale. Each tumor is characterized by specific genomic alterations that include multiple gene mutations, amplifications and/or deletions. These cancer cells interact with different components of the tumor microenvironment. Our lab focus on the correlation between genomic alterations of cancer cells, the immune infiltrate and response to therapies in women’s cancer. We are committed to precision medicine by combining our knowledge of genomic alterations of cancer cells and the immune composition of the tumor microenvironment in order to develop new target therapies. We use laser capture microdissection, next-generation sequencing, digital pathology, multiplexed immunohistochemistry and artificial intelligence to reach our goal. Currently, our main research topic is women’s cancer related to inherited BRCA1 and BRCA2 mutations. A second research topic of our lab is pathogenesis of ovarian cancer. We are investigating the cell of origin of different ovarian cancer histotypes in order to develop new screening tools and target therapies of the “silent killer”. The lab’s research is strongly oriented toward real world translation of these findings into diagnostic and therapeutic tools to improve the lives of women with cancer.
BRCA1/BRCA2 mutations in breast and ovarian cancer
Germline mutations of BRCA1/BRCA2 genes occur in up to 5% of breast cancer patients and 15% of ovarian cancers. These genes are major players in the repair of DNA double strand breaks. BRCA carriers have therefore increased sensitivity to DNA-damaging agents, such as platinum or PARP inhibitors. We recently showed a correlation between the BRCA2 genotype and response to platinum in ovarian cancer patients. Only BRCA2 carriers, harboring mutations located in the RAD51-binding domain (RAD51-BD), have prolonged treatment-free intervals and longer survival, whereas the other BRCA2 carriers did not show a survival benefit. We are currently investigating the impact of BRCA mutations on toxicity and response to chemotherapy in breast cancer patients.
Pathogenesis of high grade serous ovarian carcinoma
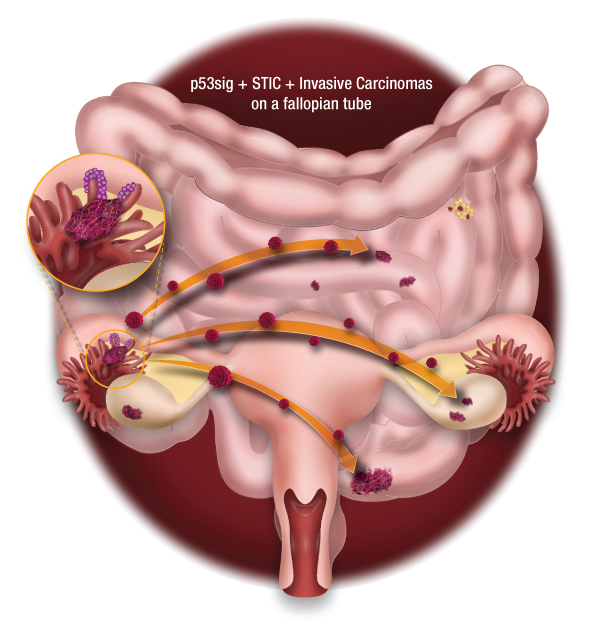
Figure 1: A proposed model of high-grade ovarian carcinoma that arise from the fimbrae of the fallopian tube. Illustration by Michael Cooper (cooper247.com)
High-grade serous ovarian carcinoma (HGSOC) is the most frequent and most lethal subtype of ovarian cancer. It is mainly diagnosed at advanced stages. In the last decade, compelling data suggested that the cell origin of HGSOC is the fallopian tube rather than the ovarian surface epithelium. Recently, we conducted extensive genomic analyses (whole-exome sequencing and copy number variation) of early tubal lesions of HGSOC (p53 signatures), serous tubal intraepithelial carcinomas (STICs), and invasive carcinomas of the fallopian tubes, ovaries and peritoneum. On average, the timing of progression from STICs to ovarian cancer was 6.5 years, with the cancer spreading to other areas quickly thereafter. Our work has provided important information about the development of HGSOC and has implications for prevention, early detection and therapeutic intervention of this disease.
