Research
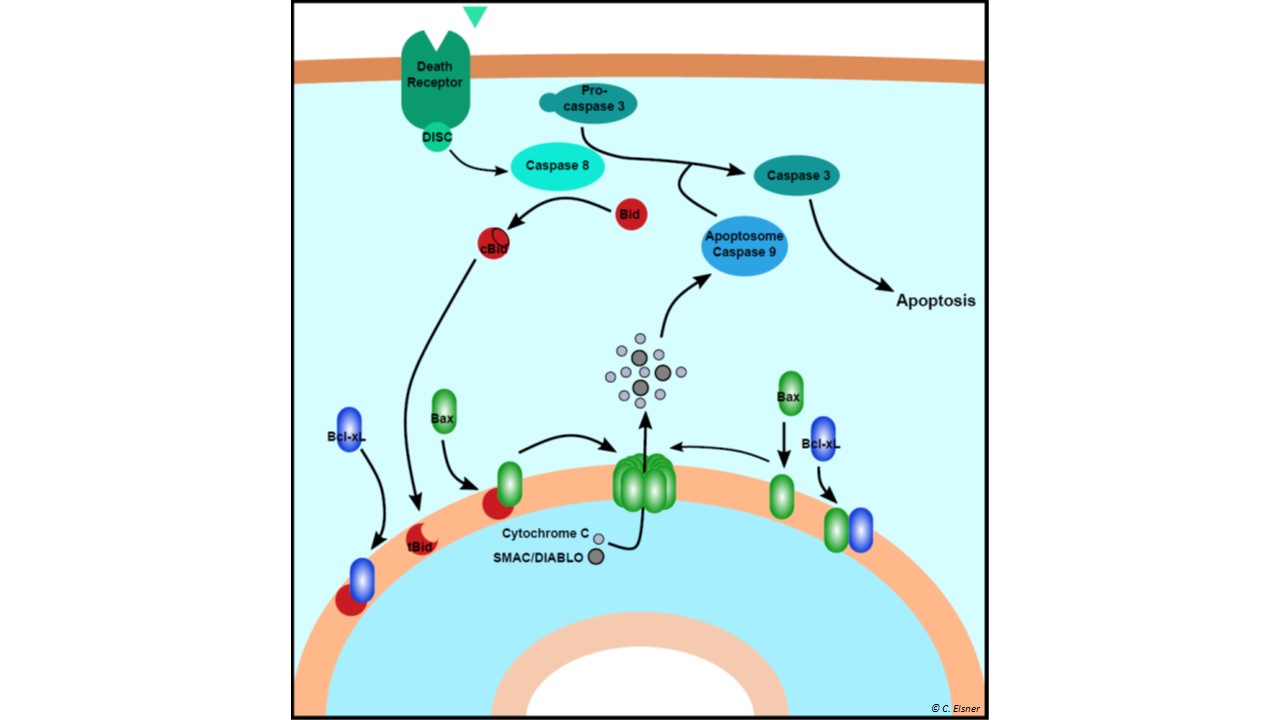
Apoptosis, a form of programmed cell death, is a vital mechanism involved in many processes in multicellular organisms. It is a tool to remove unnecessary or harmful cells without causing any inflammation. While it is important for the development of organisms and the renewal of cells, it is also a tool of the immune system. Malfunctioning of this vital process can cause cancer or Alzheimer’s disease. Therefore, a better understanding of the process is fundamental for further research to treat those diseases.
Our studies focus on the mitochondrial (intrinsic) pathway of apoptosis which is regulated by three groups of Bcl-2 family proteins. Pro-apoptotic pore formers (Bax, Bak) perforate the mitochondrial outer membrane. The pro-apoptotic BH3-only activators (Bid, Bim...) convert various signals to an activation of the pore former proteins. To avoid accidental triggering of apoptosis, anti-apoptotic inhibitors (Bcl-xL, Bcl-2) prevent pore formation. These three protein groups create a fine balance to determine if a cell starts the process of apoptosis.
To decipher the complex interaction network of the Bcl-2 family, we utilize site-directed spin labeling EPR to map out the structural changes of the involved proteins and their interactions at artificial and native membranes.
Collaborators:
Prof. Francesco GERVASIO, University of Geneva, Switzerland
Prof. Stefan RAUNSER, MPI für Molekulare Physiologie, Germany
Dr. Tobias RAISCH, MPI für Molekulare Physiologie, Germany
Grants: Sinergia Project MITOpore CRSII5_216587
Publications highlights:
The first model of one Bax dimer at the membrane:
https://www.sciencedirect.com/science/article/pii/S1097276514007795?via%3Dihub
Orthogonal labels on Bcl-2 proteins
ABC transporters are divided into importers, found exclusively in bacteria, and exporters, present in all phyla of life. Transport processes mediated by more than forty human ABC exporters fulfill vital functions in our body as they translocate an extraordinarily wide range of cargoes such as lipids, peptides, ions and drugs across lipid bilayers. A number of severe hereditary diseases including cystic fibrosis and insulin secretion disorders such as neonatal diabetes and hyperinsulinism are directly linked to ABC transporter malfunction (CFTR and SUR1, respectively), underpinning their paramount importance in human health. The human ABC exporters P-glycoprotein, ABCG2 and MRP1 act as multidrug efflux pumps in tumors and hamper effective cancer treatment.
ABC exporters minimally consist of two transmembrane domains (TMDs) each containing six transmembrane helices which protrude far into the cytoplasm and two nucleotide binding domains (NBDs), which undergo large conformational changes in response to ATP binding and hydrolysis. Transport across the membrane requires substrate binding to the inward-facing cavity, NBD closure and transition to the outward-facing (OF) state and substrate release.
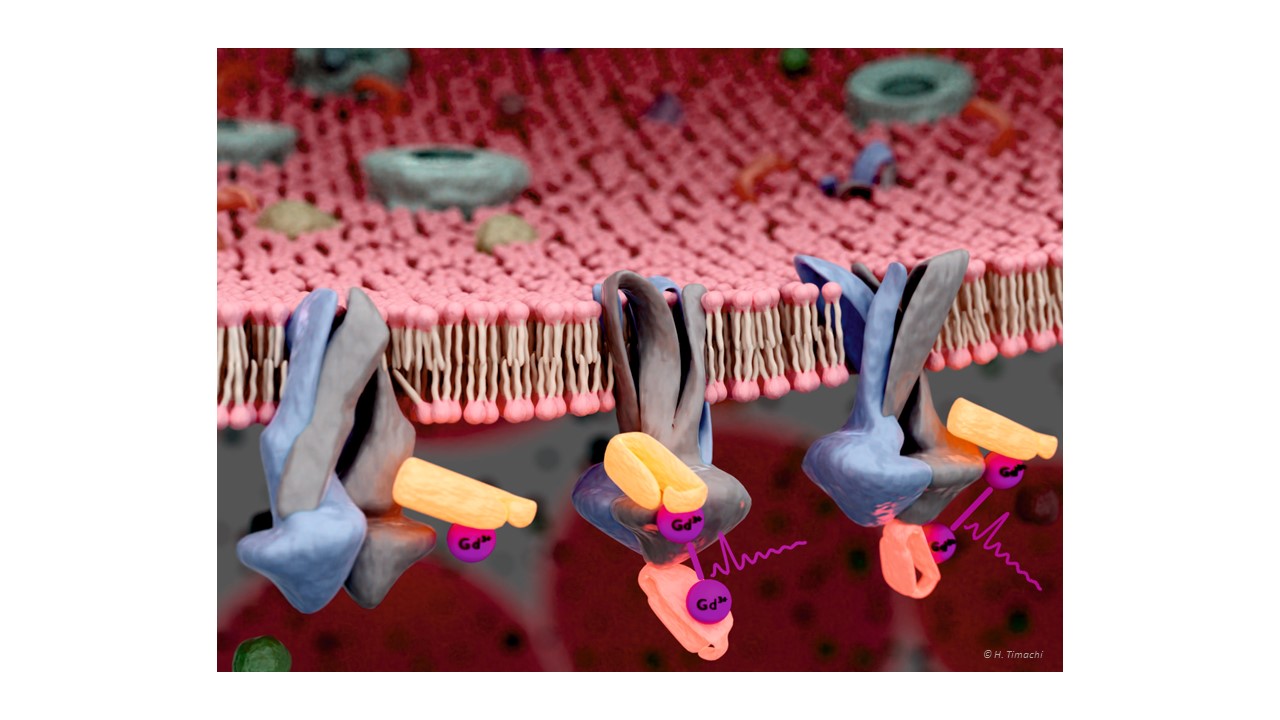
In our lab we investigate the transport cycle using spin-labeled transporters or spin-labeled nanobodies targeting transporters by in-vitro and in-cell EPR methods. Double electron electron resonance (DEER) distance measurements and Overhauser dynamic nuclear polarization techniques are particularly useful for our aim. The paramagnetic spin probes are attached to the site of interest enabling us to accurately measure the distance between pairs of spin labels and/or study water accessibility and dynamics around the spin probe.
Collaborators:
Prof. Markus SEEGER, University of Zurich, Switzerland
Prof. Lars SCHAEFER, Ruhr-University Bochum, Germany
Publication highlights:
In-cell DEER on a membrane protein with spin-labeled nanobodies:
https://www.science.org/doi/10.1126/sciadv.abn6845
Spin-labeled nanobodies as conformational reporters:
https://www.pnas.org/content/117/5/2441.full
Exploring conformational equilibria of a heterodimeric ABC transporter:

Liquid-liquid phase separation is an intriguing physico-chemical phenomenon which is related to the thermodynamic equilibrium between two liquid phases appearing in proteins’ solutions: the proteins isolated in solution coexist with molecular condensates. Emerging evidence suggests that LLPS could underlie the formation of membrane-less organelles in eukaryotic cells.
In our new project, we started to investigate the onset of LLPS of eye-lens protein γ-crystallin using the nitroxide spin labels as reporters of side chain dynamics to understand the modification of the dynamic properties of the surface residues upon formation of droplets. Distance measurements will also be used to monitor changes in structure and to follow the interactions with cosolutes and protein or DNA/RNA partners.
Collaborators:
Prof. Lars SCHAEFER, Ruhr-University Bochum, Germany
Prof. Martina HAVENITH, Ruhr-University Bochum, Germany
Prof. Takuji ADACHI, University of Geneva, Switzerland
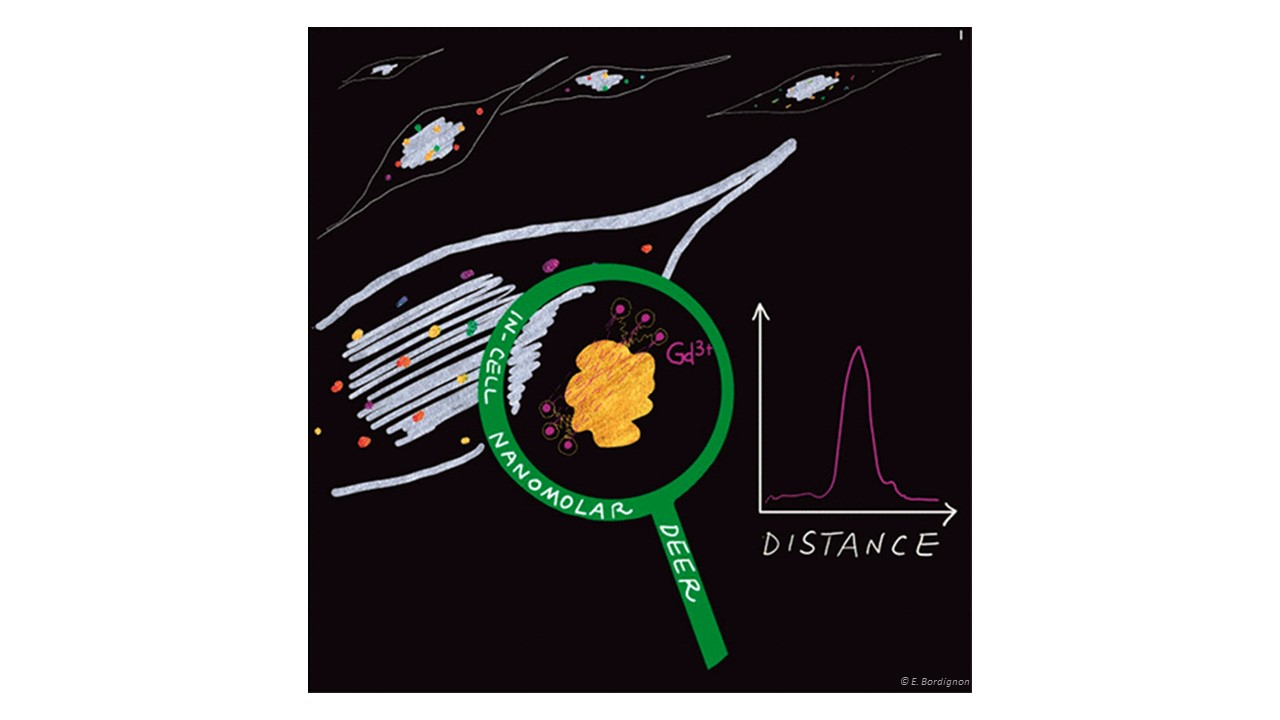
Site-directed spin labeling EPR in combination with pulse dipolar spectroscopy allows investigating structure and conformational changes of proteins in vitro with high sensitivity. Proteins” size and environment do not impact the sensitivity of PDS methodology therefore, we can investigate small peptides (down to a few amino acids) and large proteins (up to Megadalton, see toxin project) and we can use membrane mimicking environments such as micelles, liposomes, nanodiscs or inside out vesicles of E. coli to stabilize membrane proteins.However, we aim to get insights into structure, interactions and function of a protein in its physiological environment, namely the cell. To this end, we label recombinantly produced water soluble proteins with biocompatible labels and electroporate them into cells at physiological concentration (down to hundreds of nanomolar per cell). By extracting distance constraints in cells, we can create coarse grained models of their conformational changes and characterize their interactions with other partners.
Towards the goal of watching a membrane protein move in its membrane bilayer, we pioneered the use of spin-labeled nanobodies as conformational reporters of membrane proteins in cells.
For Bcl-2 proteins, we opted to use freshly isolated mitochondria from mice liver to provide the physiological milieu where oligomerization of Bax occur (see apoptosis project) at the onset of apoptosis.
Publication highlights:
In-cell DEER on a membrane protein with spin-labeled nanobodies:
https://www.science.org/doi/10.1126/sciadv.abn6845
Nanomolar DEER sensitivity in cell:
https://pubs.acs.org/doi/abs/10.1021/acs.jpclett.1c00048
Spin-labeled nanobodies as conformational reporters for membrane proteins
Toxins are of the general biochemical interest as they can be used as biological weapons (e.g. biopesticides) or for biopharmaceutical applications. Tc toxins are 1.7 MDa protein complexes that are found in insect- and human-pathogenic bacteria. The complex consists of three subunits: the 1.4 MDa TcA pentamer, which mediates target cell association,membrane insertion and toxin translocation, and two smaller subunits, TcB and TcC, which form a 250 kDa cocoon that encapsulates a toxic enzyme. The transformation of the toxin from a prepore to a pore state and the concomitant release of the enzyme is pH-driven.

We investigate pH-driven kinetics of the prepore-to-pore transition of a Tc toxin in vitro by continuous wave EPR and DEER spectroscopy. In addition, we study the allosteric coupling induced by point mutations. We use an integrative approach combining EPR with cryo-EM and single molecule fluorescence methods.
Collaborators:
Prof. Stefan RAUNSER, Max Planck Institute of Molecular Physiology, Dortmund, Germany
The combination of silver oxide (AgOx) nanoislands deposited on a titanium oxide (TiOx) support layer could potentially be employed for the treatment of healthcare-acquired infections. Under light irradiation, the plasma-deposited coatings show increased antimicrobial properties due to the production of reactive oxygen species (ROS). Additionally, metal oxide plasma coatings can modulate the biocompatibility of the material, by characteristics like wettability and cell adhesion. Despite these promising features, a thorough characterization of the ROS delivery is needed for possible future biomedical applications.
To explore the radical release from plasma-deposited materials, we employ spin traps (e.g., DMPO: 5,5-Dimethyl-1-Pyrroline-N-Oxide) which form stable paramagnetic species in the presence of ROS. By EPR, we can trace the production of radicals in different plasma functionalized materials and characterize the type of ROS generated by the metal oxide catalyst coating.
Collaborators:
Dr. Paula Navascués, EMPA, Switzerland
