Recherches (en anglais)
CROSS-COUPLINGS AND FUNCTIONALIZATIONS OF ALKENES:
making bonds (selectively)
One of our goal is to generate complexity by selective functionalization and cross-coupling reactions of readily available building blocks. Some of our recent methods are highlighted below.

Among other examples, we identified two complementary Ni catalysts for a Kumada cross-coupling reaction between vinyl magnesium bromide and readily available vinyl phosphates, affording a broad diversity of 2-aryl and 2-alkyl 1,3-dienes.

By simple adjustment of the relative stoichiometry of the reagents, we discovered a one-pot [Ni/Mg] orthogonal tandem catalytic polymerization protocol in which the Ni-catalyzed Kumada cross-coupling, that produces 1,3-dienes in situ, is followed by a Mg-initiated polymerization process that affords new 1,4-cis-polydienes. The high functional diversity of the monomers obtained allows to easily tune the thermal properties of the corresponding materials, thus giving access not only to elastomeric polymers (classical for 1,4-cis-polydienes) but also to crystalline polymers (generally observed for 1,4-trans-polymers).
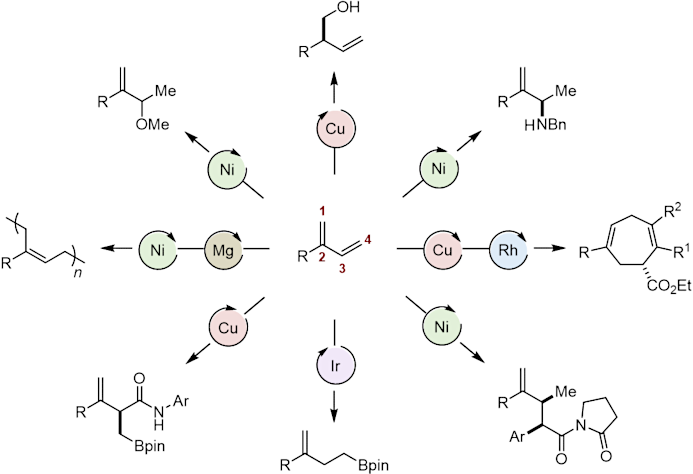
Subsequently, we started to explore the use of branched dienes as a platform for the development of a multitude of complementary catalytic and selective protocols such as a Cu-catalyzed enantioselective 1,2-borylation, a Ni-catalyzed enantioselective intermolecular hydroamination, a two-step enantioselective cyclopropanation/[5 + 2] cycloaddition catalytic sequence that gives access to a variety of enantioenriched seven-membered rings, a Ni-catalyzed regiodivergent and stereoselective hydroalkylation using unstabilized nucleophiles … etc…
