- Multicatalytic Stereoselective Synthesis of Highly Substituted Alkenes by Sequential Isomerization/Cross-Coupling Reactions
Romano, C.; Mazet, C.
J. Am. Chem. Soc. 2018, 140, 4743-4750
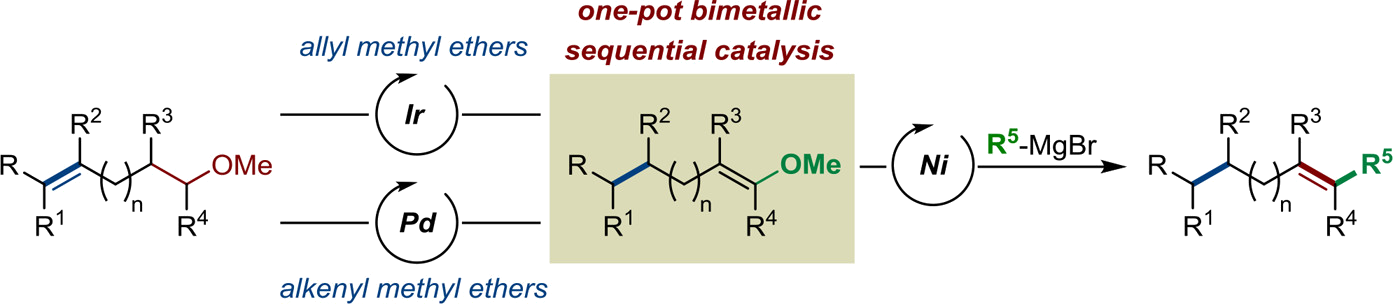
Starting from readily available alkenyl methyl ethers, the stereoselective preparation of highly substituted alkenes by two complementary multicatalytic sequential isomerization/cross-coupling sequences is described. Both elementary steps of these sequences are challenging processes when considered independently. A cationic iridium catalyst was identified for the stereoselective isomerization of allyl methyl ethers and was found to be compatible with a nickel catalyst for the subsequent cross-coupling of the in situ generated methyl vinyl ethers with various Grignard reagents. The method is compatible with sensitive functional groups and a multitude of olefinic substitution patterns to deliver products with high control of the newly generated C=C bond. A highly enantioselective variant of this [Ir/Ni] sequence has been established using a chiral iridium precatalyst. A complementary [Pd/Ni] catalytic sequence has been optimized for alkenyl methyl ethers with a remote C=C bond. The final alkenes were isolated with a lower level of stereocontrol. Upon proper choice of the Grignard reagent, we demonstrated that C(sp2)-C(sp2) and C(sp2)-C(sp3) bonds can be constructed with both systems delivering products that would be difficult to access by conventional methods.
DOI : 10.1021/jacs.8b02134
archive ouverte unige:103401
