Membrane fission and dynamin
The clathrin-coated pits are separated from the membrane by a process called fission. As lipid bilayers are auto-sealable, fission is not a spontaneous event. This important step for membrane dynamics in cells is difficult to study as it is very transient.
Only one protein has been so far shown to have a clear mechano-enzymatic activity: dynamin. Dynamin is able to generate and break membrane tubules by wrapping its helix around the tubule and then constricting it through twisting upon hydrolysis of GTP. We are currently measuring important mechanical parameters such as the torque, the angular step and the energy yield of dynamin while it is exerting its twisting activity to unravel the exact mechanism by which dynamin fission occurs.
Dynamin
Dynamin is a GTPase that has been implicated genetically and biochemically in the fission of membranes in living cells (Praefcke and McMahon, 2004).
It polymerizes into a helicoidal collar (14 dimers per turn) at the neck of endocytic buds (see figure 1).
In vitro, it was shown that dynamin could tubulate negatively charged membranes by assembling its helix around the membrane. GTP hydrolysis promotes the constriction of the helix and of the membrane tubule underneath (Danino et al., 2004; Sweitzer and Hinshaw, 1998).
This constriction is thought to be responsible for the fission of the membrane, and is mediated by a twisting mechanism (see figure 1 and Roux et al., 2006). Dynamin-mediated fission occurs at the plasma membrane but also probably at the Trans-Golgi network. Even though different processes have been proposed for membrane fission, dynamin is the only protein shown to have a direct mechanical role in fission.
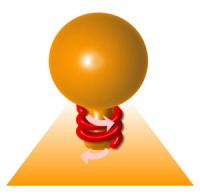 figure 1: Dynamin (red) polymerizes into a helical collar at the neck of endocytic buds, and constricts by torsion (pink arrows) in order to break membrane.
figure 1: Dynamin (red) polymerizes into a helical collar at the neck of endocytic buds, and constricts by torsion (pink arrows) in order to break membrane.
Membrane fission
In the lab, we are interested in understanding how membrane can be fissioned.
By using a micromanipulation technique that combines micropipettes and optical tweezers, we are able to extract thin membrane tubes from Giant Unilamellar Vesicles (see figure 2).
We can measure and control their diameter, and measure forces applied by dynamin to the membrane with optical tweezers. We recently showed that dynamin polymerization sharply depends on membrane curvature at low bulk concentration, and this phenomena depends strongly on the squeezing force generated by dynamin (Roux et al. 2010).
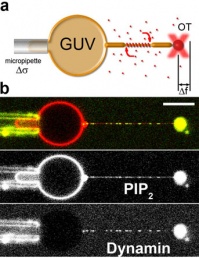 figure 2: Forces and radius measurement during dynamin/membrane interactions.
figure 2: Forces and radius measurement during dynamin/membrane interactions.
a) Optical tweezers (OT) and micropipette set-up to measure the force (Df) and the tension (Ds) which allow the calculation of the radius.
b) a typical experiment where dynamin in solution binds to the membrane tube as seen by confocal microscopy.
From Roux et al. 2010.
We are currently studying the torsion and fission mediated by dynamin in this system. We hope to gain better insights into the energetics and physics of membrane fission.
