Glucose / Lipid metabolism
1. Glucose/insulin clamp
The euglycemic hyperinsulinemic clamp is the "gold standard" technique to determine insulin sensitivity of an organism in vivo. During the clamp period, blood glucose and insulin levels are stably fixed: the amount of glucose infused per minute (or glucose infusion rate = GIR) needed to maintain normal glycemia in the context of hyperinsulinemia is a direct measurement of insulin sensitivity.
In combination with the use of different glucose tracers, this technique allows determining the action of insulin on glucose metabolism in the whole organism or in a tissue-specific manner.
Namely:
- The hepatic glucose production (HGP) or endogenous glucose production (EndoRa), basal or insulin-stimulated.
- Glucose turnover (Rd), basal or insulin-stimulate.
- Tissue-specific glucose uptake under insulin stimulation (in skeletal muscles, adipose tissue depots, heart, brain, etc.)
Technique
The euglycemic hyperinsulinemic clamp can be performed in anaesthetized or awake animals (rat, mouse).
The procedure for performing clamps in awake and freely moving animals is the following: a catheter is placed in the jugular vein 7 days before the experiment.
The day of the experiment, animals will be either fasted for 5 hours (mouse) or overnight (rat). The rate of insulin infusion (2.5 to 20mU/kg.min-1) is adapted to the metabolic parameters of interest: a low dose of insulin allows evaluating the sensitivity to insulin in the liver, while a high dose is better suited to evaluate insulin sensitivity in tissues such as skeletal muscles.
The choice of the dose of insulin should also take into account the age, sex, adiposity, and/or genotype of the animal.
The equipment (pumps, infusion system) also enables to perform hyperglycemic clamps (to test the secretory capacity of the pancreas) and hyperinsulinemic hypoglycemic clamps (to evaluate counter-regulatory systems).
- Infusion system: Instech Laboratories Inc. (http://www.instechlabs.com/)
- Pumps: KdScientifics (http://www.kdscientific.com/ ); and Harvard Apparatus Elite II (http://www.harvardapparatus.com/ )
Planning
A preliminary meeting with the managing team will allow to establish the optimal conditions for the experiment (insulin infusion, selected tracers, tissues to analyze, number of animals).
Once the condition are established, the principal investigator of the applicant team should agree with the prospected study by email.
At this point, the experiment is scheduled. Applications should be sent several weeks prior to the actual experiments.
Please contact Dr. Christelle Veyrat-Durebex to schedule a meeting.
Use of this technique should be included in your Animal Experimentation Authorization (e-tierversuche) and approved by the canton Veterinary Office (SCAV). The team can help you to obtain this authorization (protocols and experimenter already approved by the SCAV; Protocol will be provided on request).
Data will be processed and provided as electronic documents to the user. Aid in the interpretation of the results is also proposed. For more information, please contact Dr. Christelle Veyrat-Durebex.
Procedure
Animals:
We highly suggest a minimum of 8 animals per group. In some cases (e.g. severe obesity), an additional smaller cohort may be necessary to complete the experiment.
If special diets or drinks are needed, these should be provided by users.
Reagents:
All reagents are provided by the plateform.
Timing (awake mice):
Week 1-3: Surgery (2 to 3 animals/day).
The recovery from surgery will be followed by a measurement of body weight. The catheters will be washed up every two days.
Week 2-4: Clamps are performed (2 to 3 animals/day)
Week 5-6: Blood and tissue samples are processed for radioactivity measurements. Results are handled by the platform to verify for absence of any aberration.
Guidelines
Animal health status
Health status report is requested for any animal from outside the CMU. A recent health status report (related to the specific rooms that host the animals) must be obtained by the user from the head of his Institute’s animal Core Facility and be sent before the first meeting with the platform team. No animal will be allowed without the approval to Dr Maelle Le Pottier, our institution’s veterinary officer. Any animal with problems (injury, infection, abnormal behavior) will be sacrificed.
Priority in experiments
The platform aims at providing access to the largest number of users possible. Priority access is on a “first come-first served” basis. Therefore, last-minute requests might be honored with a significant delay. However, some flexibility, which might depend on practical aspects, can be considered.
The applicant team should agree to provide the required animals for the planned experiments, and to pay the appropriate fees.
The applicant also acknowledges and accepts that the final price and total number of animals required to complete the test may vary according to difficulties encountered, specifically with some rodent models.
Data
All data strictly belong to the investigator and his institution. Thus, the data cannot be used by the platform personnel for personal or institutional research purposes, unless a formal written agreement of collaboration is established between the two parties.
2. Metabolic tests
Different metabolic tests could be realized by the core facility. For each of them, please contact Dre for details and planning.
Injections and blood sampling are realized by the core facility according to protocols established by the users.
6 to 8 mice/group are recommended.
Period of fasting should be planned and realized by the users, as a posteriori analyses of blood samples.
Glucose homeostasis
- Glucose tolerance test (ipGTT/ivGTT/oGTT)
- Insulin tolerance test (ipITT/ivITT)
- Pyruvate tolerance test (ipPTT)
Protocols as determined by the users
10CHF/mouse (12 mice) (12 CHF for additional mouse)
- Glucose tolerance test with a 2-deoxyglucose tracer (ip or ivGTT / 2DG uptake)
Allows determination of glucose uptake specifically in each tissues during a glucose tolerance test by simultaneous injection of radiolabeled 2-DG.
150CHF / mouse (12 to 16 mice)
- Measurement of glucose uptake in basal condition with a 2-deoxyglucose tracer (iv).
Allows determination of glucose uptake specifically in each tissue (with / without a 4hrs fast) through radiolabelled 2-DG IV injection.
120CHF / mouse (12 to 16 mice)
- Measurement of endogenous glucose production (EndoRa) and glucose uptake by peripheral tissues.
Measures hepatic glucose production (HGP or EndoRa) and tissue glucose uptake after a 4-hour fast, by infusion and injection of 2 radiolabelled tracers, on anesthetized mice.
250CHF / mouse (12 mice)
Lipid homeostasis
- Lipid tolerance test (oLPT): oral olive oil gavage, followed by tail vein blood sampling over a 6hrs period (sampling after 1.5, 3, 4.5 and 6hrs).
A fasting of 6 to 18hrs is recommend before the experiment.
12CHF/mouse (12 mice) (15 CHF for additional mouse)
For more information, please contact Dr. Christelle Veyrat-Durebex.
- Lipogenesis/ de novo lipogenesis
It is possible to quantify lipogenesis and de novo lipogenesis from glucose in the liver and various adipose tissues, using two different radiolabeled tracers (tritiated H2O, and 14C-U-glucose).
Different protocols are proposed for the mouse and / or the rat, depending on the users' request, involving or not the placement of a venous catheter for 7 days before infusion of the tracers.
7-8 animals per group are advised.
The use of these techniques must be included in your authorization for animal experiments issued by the DGS. The team can help you obtain this authorization, especially in case of chronic catheterization.
Price (12 to 16 mice):
CHF 60 / mouse (lipogenesis)
120 CHF / mouse (de novo lipogenesis - injection bolus)
250 CHF / mouse (de novo lipogenesis - infusion)
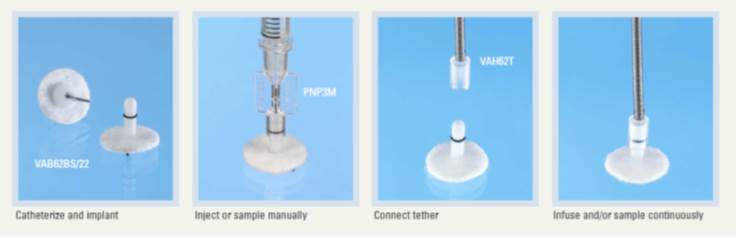
3 gluco-sensor telemetry.
The platform is equipped with a telemetry system from the company DSI ™ allowing the simultaneous recording of 12 cages (https://www.datasci.com/).
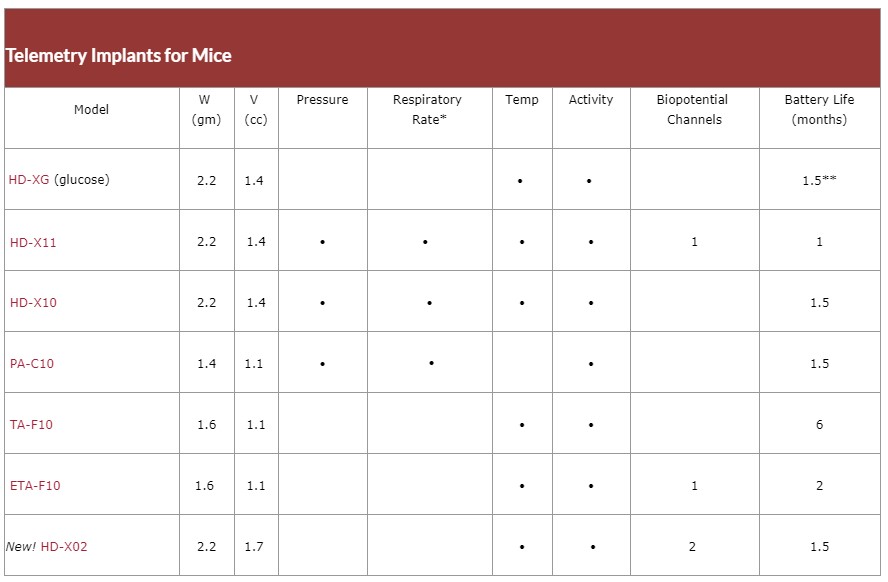
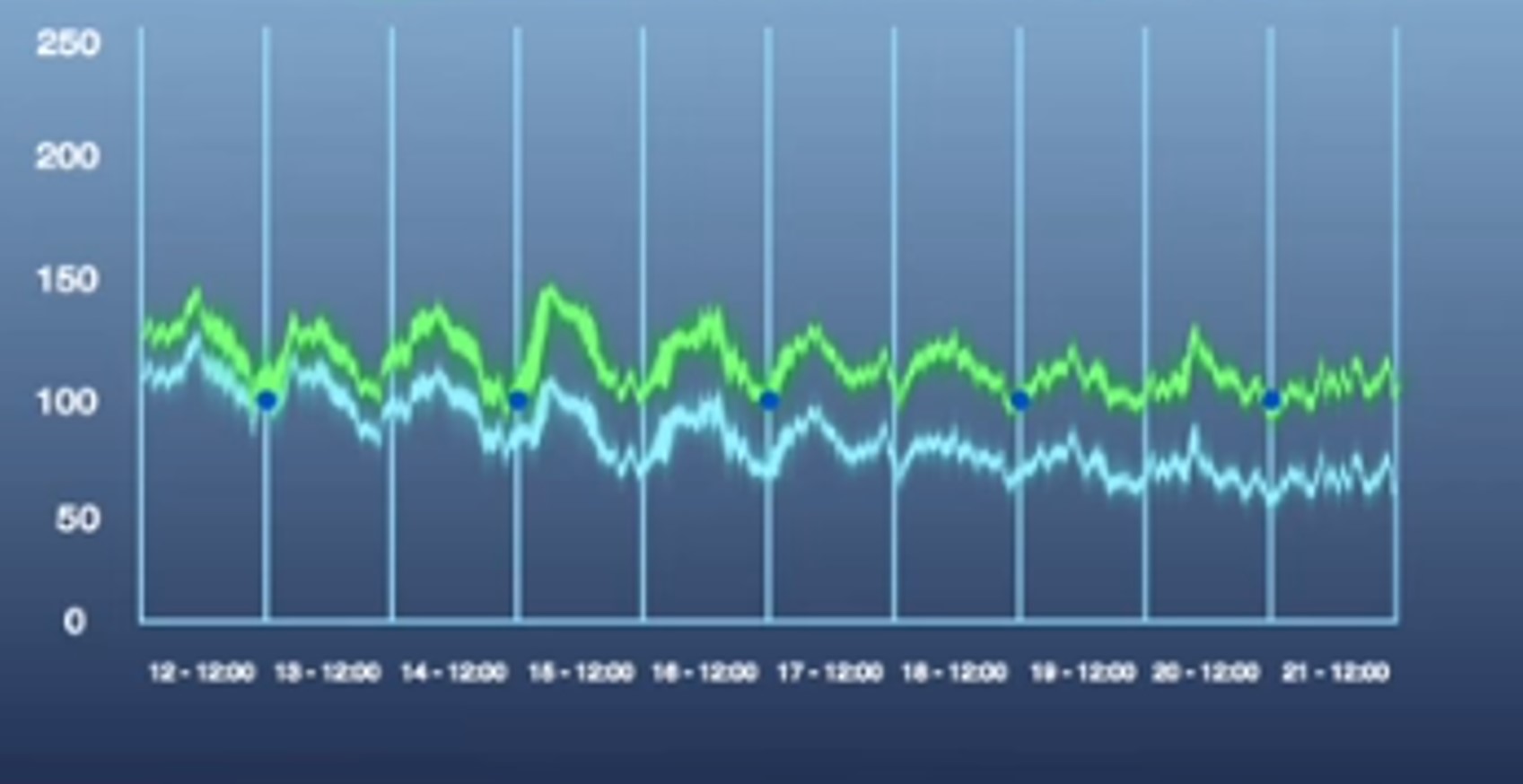
The animals (rats and mice) will be implanted with the various sensors developed by this company, allowing to record continuously over 1 to 2 months:
• Physical activity
• Body temperature
• Blood pressure
• Respiratory rate
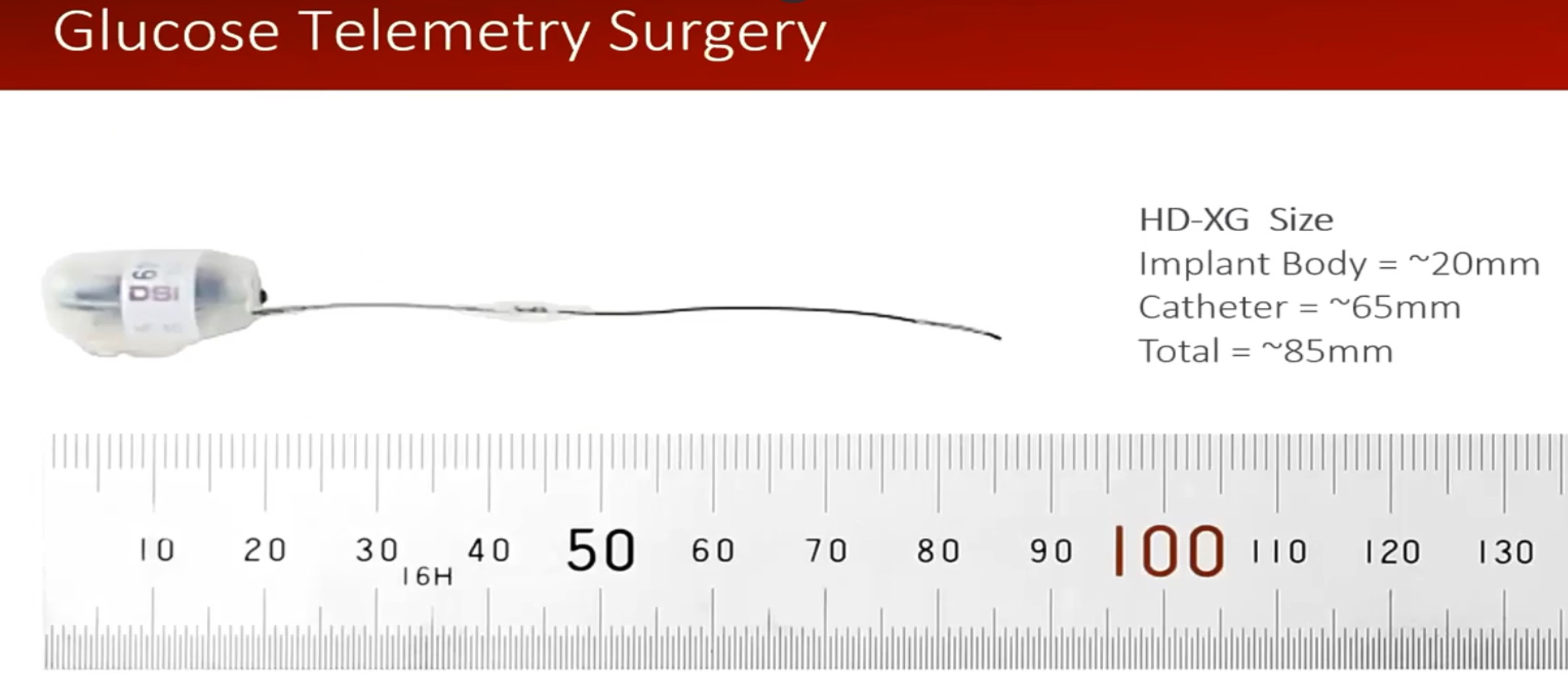
• But especially with the new HD-XG sensor, blood glucose.
Planning of the study
A preliminary meeting with the team will allow you to establish the optimal conditions for your experiences. The use of this technique must also be included in your authorization for animal experiments issued by the DGS. The team can help you obtain this authorization. For more details, please contact Dr. Christelle Veyrat-Durebex.
Procedure
The mice will be implanted by the staff of the platform, under anesthesia with isoflurane and followed, post-surgery for 6 days. However, users can start or stop the recordings at any time depending on their protocol.
Example of sensors for mice:
For users
Sensors are on charge of users.
Bring the animals and their special food / drink used. Please contact Christelle Veyrat-Durebex to arrange the first appointment and the booking period.
Animal health status
A health status certificate is required for any animal coming from outside the CMU. No animal will be admitted without the approval of Dr. Maelle Le Pottier.
Priority in experiments
The platform aims to provide access to as many users as possible. Priority access is therefore "first come, first served". As a result, last-minute requests may be delayed. However, some flexibility, which may depend on practical aspects, may be considered.
The applicant also acknowledges and agrees that the final price and the total number of animals required to complete the experiment may vary depending on the difficulties encountered, particularly with certain rodent models.
Data
All data strictly belongs to the user team and its institution.
