Tracking Ceramides: Fluorescent KSR1 Probes
SUMMARY
Ceramides regulate phagocytosis; however, their exact function remains poorly understood.
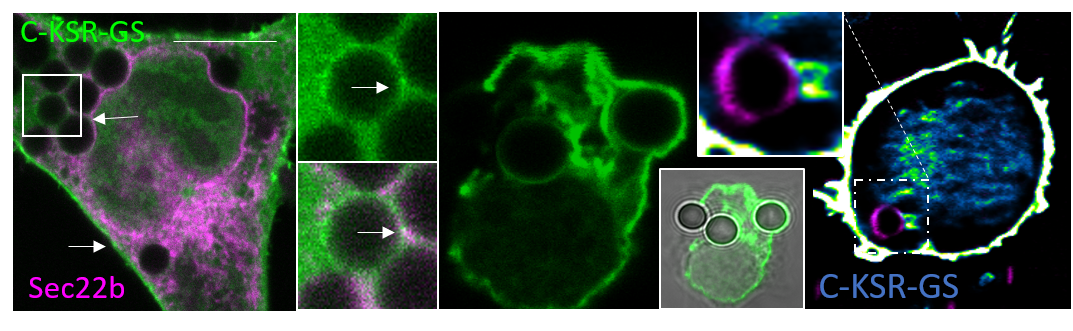
In this article, led by GCIR Professor Paula Nunes-Hasler, her team sought (1) to develop genetically encoded fluorescent tools for imaging ceramides, and (2) to use them to examine ceramide dynamics during phagocytosis. Fourteen enhanced green fluorescent protein (EGFP) fusion constructs based on four known ceramide-binding domains were generated and screened. While most constructs localized to the nucleus or cytosol, three based on the CA3 ceramide-binding domain of kinase suppressor of ras 1 (KSR1) localized to the plasma membrane or autolysosomes. C-terminally tagged CA3 with a vector-based (C-KSR) or glycine-serine linker (C-KSR-GS) responded sensitively and similarly to ceramide depletion and accumulation using a panel of ceramide modifying drugs, whereas N-terminally tagged CA3 (N-KSR) responded differently to a subset of treatments. Lipidomic and liposome microarray analysis suggested that, instead, N-KSR may preferentially bind glucosyl-ceramide. Additionally, the three probes showed distinct dynamics during phagocytosis. Despite partial autolysosomal degradation, C-KSR and C-KSR-GS accumulated at the plasma membrane during phagocytosis, whereas N-KSR did not. Moreover, the weak recruitment of C-KSR-GS to the endoplasmic reticulum and phagosomes was enhanced through overexpression of the endoplasmic reticulum proteins stromal interaction molecule 1 (STIM1) and Sec22b, and was more salient in dendritic cells. The data suggest these novel probes can be used to analyze sphingolipid dynamics and function in living cells.
Full article: https://doi.org/10.3390/ijms25052996
WHY is it important?
Ceramides are essential bioactive lipids crucial for cell maintaining cell membranes, metabolism, cardiovascular health, and immunity. Despite their recognized significance, studying these elusive lipids has been challenging since current methods used to measure them are expensive and technically complex. This study introduces genetically encoded fluorescent probes that offer a simple, cost-effective solution to track changes in cellular ceramide levels. The authors also uncovered intriguing differences in cellular ceramide dynamics during phagocytosis in dendritic cells as compared to another cell model, suggesting that investigating ceramides might help us better understand how these important immune cells ingest and process pathogens to alert the adaptive immune system. These new user-friendly tools will be of great help to researchers aimed at gaining a deeper understanding of ceramides in normal biological functions, their implications in diseases and in the search for new therapeutic strategies based on ceramide biology.
7 Mar 2024