A crucial interplay between two signaling pathways
Proper cellular functions require the precise coordination of numerous signaling pathways. As a vast communication network, they ensure that every cellular function runs smoothly. Among these pathways, store-operated calcium entry (SOCE) and endoplasmic reticulum stress (ER stress) play pivotal roles by respectively governing calcium balance and protein stability within cells. Understanding how they work is key, since their dysfunction leads to severe diseases such as neurodegenerative disorders and cancer.
Direct link between the two pathways discovered
Intrigued by the fact that both signaling axes involve a cellular organelle called the endoplasmic reticulum and can be experimentally inhibited by the same drug, Dr Amado Carreras-Sureda, in collaboration with other researchers from the UNIGE laboratories of Pr Nicolas Demaurex and Pre Maud Frieden, began to explore their possible interaction. Using various cellular models, the scientists uncovered a crucial cellular interaction between SOCE and ER stress.
They discovered that the protein IRE1 (in green in the Figure below), known for its role in ER stress, interacts with a protein involved in SOCE, STIM1 (in red in the Figure below). This direct link between the two proteins was shown to enhance specific contact points between the endoplasmic reticulum and the cell membrane, ultimately boosting the entry of calcium (purple dots in the Figure below) and the efficiency of SOCE. Their experiments also revealed the importance of this interplay for the activation of some immune cells, T cells, and for the regeneration of muscles.
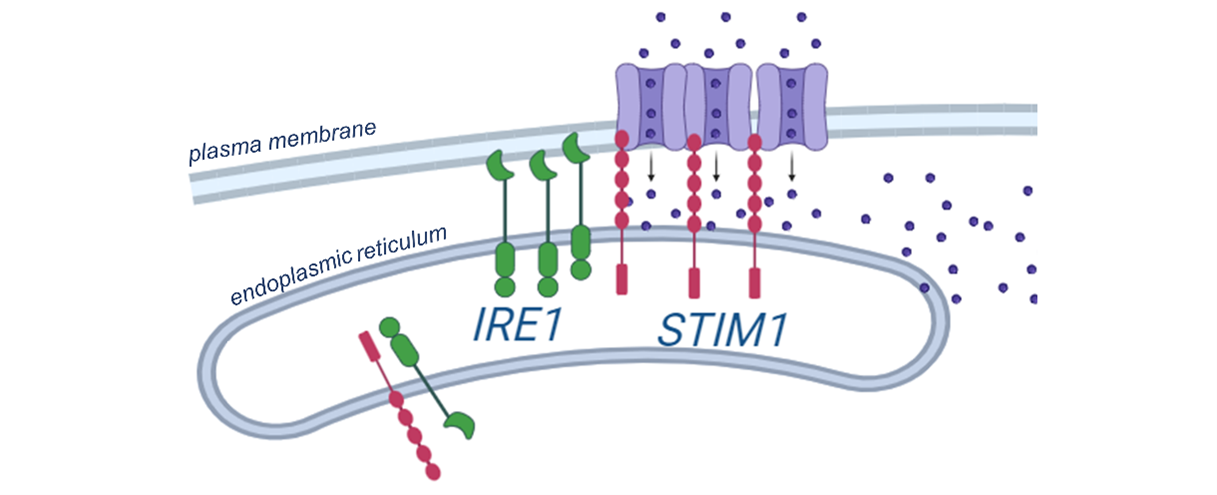
The direct contact between the IRE1 protein (in green) and the STIM1 protein (in red) proves a crucial interplay between the store-operated calcium entry (SOCE) and the endoplasmic reticulum stress. © adapted from the graphical abstract in Carreras-Sureda et al. 2023
Potential implications
This discovery illustrates the sophistication of cellular communication, demonstrating how different proteins collaborate to ensure the smooth functioning of cells. The repercussions of this research extend beyond cellular mechanics, offering promising avenues for future therapeutic strategies, particularly in the realm of cancer immunotherapy.
