Tissue Engineering and Organ Regeneration
Type 1 diabetes mellitus is an autoimmune disease characterised by the irreversible destruction of insulin-producing β-cells. Although insulin therapy remains the standard treatment for uncomplicated diabetes, the physical, psychological, and financial burden associated with the long-term complications of this disease remains a serious concern. Intrahepatic islet transplantation has unequivocally demonstrated the effectiveness of islet replacement therapy by temporarily restoring insulin independence and improving glycaemic control to near-normal levels. However, this approach faces several challenges.
Immediately after transplantation, a large portion of the islets is lost due to an immediate inflammatory reaction, which can destroy up to 70% of the transplanted islets. Additionally, insufficient blood supply deprives the islets of oxygen and nutrients, leading to cell death. Beyond these immediate threats, transplanted islets face long-term challenges. The liver, where islets are usually transplanted, does not replicate the natural environment of the pancreas. This discrepancy complicates proper islet function and leads to their gradual degradation over time. Finally, the scarcity of high-quality human donor islets limits availability, and the requirement for lifelong immunosuppression introduces side effects that can harm both the graft and the recipient.
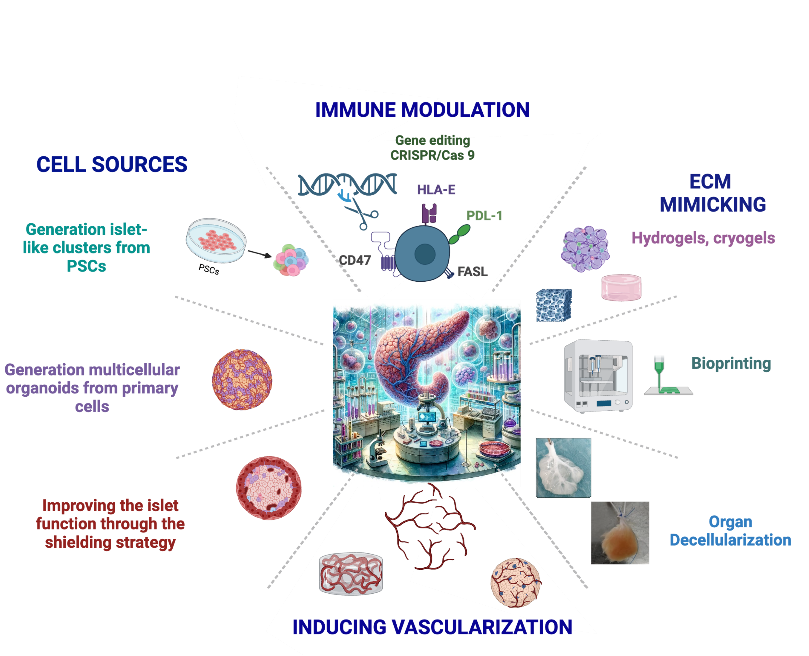
To overcome these obstacles, we are exploring innovative bioengineering strategies, including the development of alternative sources of endocrine cells and the creation of technologies that mimic the natural environment of islets.
RESEARCH AIMS
We are committed to developing an Advanced Therapy Medicinal Product (ATMP) capable of curing type 1 diabetes. To achieve this ambitious goal, we are pursuing several interconnected strategies:
- Developing unlimited sources of insulin-producing cells derived from pluripotent stem cells (iPSCs and hESCs)
- Generation of functional islet organoids
- Bioengineering scaffolds and biomaterials
- Vascularization strategies
- Immune modulation and tolerance induction / Generating hypoimmunogenic pluripotent stem cells to avoid rejection of their derivatives
Our ultimate goal is to develop a stable, long-term cure for type 1 diabetes. By generating functional insulin-producing cells, recreating the natural islet microenvironment using advanced biomaterials, and inducing immune tolerance, we aim to overcome the current limitations of islet transplantation and cell therapy.
CORE EXPERTISE
1. Stem Cell Differentiation & 3D Organoid Technology
- Directed differentiation of iPSCs and hESCs into insulin-producing beta-like cells.
- Development of 3D islet organoids mimicking the native islet microenvironment.
2. Advanced Bioengineering of Scaffolds and Biomaterials
- Design and fabrication of prevascularized hydrogel and cryogel scaffolds.
- Application of organ decellularization techniques to create ECM-rich scaffolds that retain native biochemical and biomechanical cues.
3. Engineering Functional Microvasculature
- Integration of recipient-derived human endothelial cells to build robust, organized vascular networks within engineered constructs.
- In vivo modeling of vascularization and host-graft vascular integration to promote engraftment and long-term survival.
4. Immune Modulation and Tolerance Induction
- Incorporation of immune-modulating molecules and immunoregulatory cells into scaffold platforms.
- Application of genetic engineering and immune cloaking strategies to create hypoimmunogenic insulin-secreting cells and constructs.
5. Translational & Preclinical Validation
- Deployment of in vitro and in vivo models to evaluate cell survival, function (e.g., insulin secretion), and immune protection post-transplantation.
SELECTED PUBLICATIONS
- Bellofatto K, Lebreton F, Hassany M, Hanna R, Bignard J, Marteyn A, Mar Fonseca L, Campo F, Olgasi C, Wolf-van Bürck L, Honarpisheh M, Martinez de Tejada B, Follenzi A, Citro A, Piemonti L, Thaunat O, Seissler J, Compagnon P, Cohen M, Berishvili Berney E, VANGUARD consortium, Bioengineering of the implantable vascularized endocrine constructs for insulin delivery suitable for clinical upscaling bioRxiv 2025.04.19.647461;
- Lebreton F, Hanna R, Wassmer CH, Bellofatto K, Perez L, Othenin-Girard V, de Tejada BM, Cohen M, Berishvili E. Mechanisms of Immunomodulation and Cytoprotection Conferred to Pancreatic Islet by Human Amniotic Epithelial Cells. Stem Cell Rev Rep. 2022 Jan;18(1):346-359.
- Oliveira Almeida Fonseca LDM, Lebreton F, Wassmer C-H, Berishvili Berney E. Generation of Insulin-Producing Multicellular Organoids. Methods in molecular biology 2023;2592:37‑60.
- Berishvili E, Piemonti L, de Koning EJP, Lindstedt S, Scholz H, Scott WE, Auxenfans C, Johnson P, Martin DE, Gunther P, Mey D, Potena L, Thaunat O. ESOT Roadmap for Advanced Therapy Medicinal Products in Transplantation: Navigating Regulatory Challenges to Enhance Access and Care. Transpl Int. 2024 Oct 14;37:13485.
- Lebreton F, Lavallard V, Bellofatto K, Bonnet R, Wassmer C-H, Perez LL, Kalandadze V, Follenzi A, et al. Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nature Communications 2019;10(1):4491.
 3 Jul 2025
3 Jul 2025
