Always-on variants with severe effects in GNAO1 encephalopathies
Small but important, G proteins enable cell communication by translating external cues from receptors on the cell surface into the complex language of cellular responses. Among them, the GNAO1 protein plays a crucial role in brain signaling.
The study of mutations involved in GNAO1 encephalopathies…
GNAO1 encephalopathies are rare brain disorders caused by mutations in the GNAO1 gene that encodes for the Gαo protein. Scientists still know very little about how different mutations lead to a variety of symptoms, ranging from mild developmental delays to severe movement difficulties and seizures. In a recent study published in Science Signaling, researchers from the laboratory of Prof. Vladimir Katanaev examined blood samples from three patients with severe symptoms to understand how their GNAO1 mutations disrupt brain and cell function.
… reveals “always-on” variants
The study revealed that these GNAO1 mutations alter the normal signals required for RNA production, leading to the loss of an entire region that helps Gαo proteins to change shape when needed, the Switch III. Through close teamwork, the researchers discovered that this generates “always-on” versions of the Gαo protein.
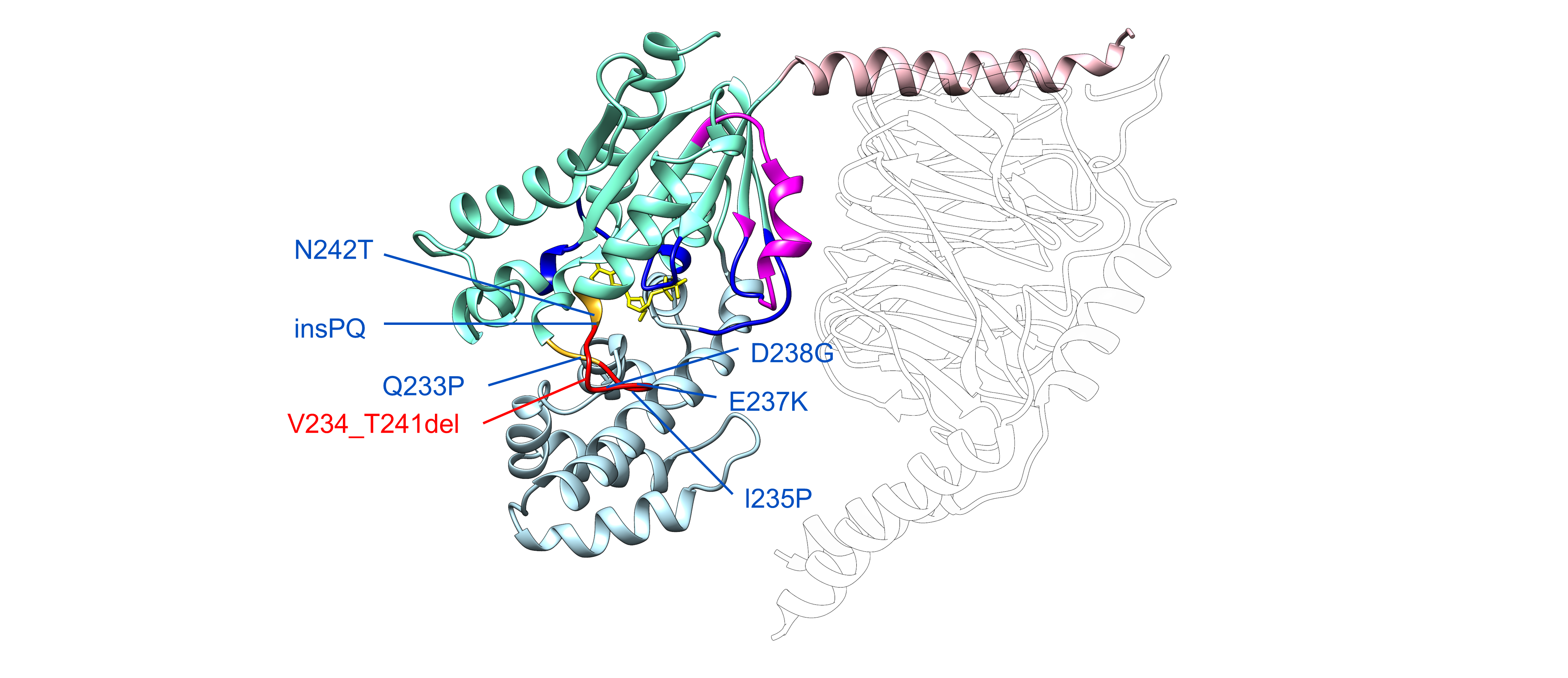
The different GNAO1 mutations (texts in blue) lead to the loss of an entire region that helps Gαo proteins to change shape when needed, the Switch III (in red), generating “always-on” Gαo variants that have new, harmful features. © Adapted from the Figure 2 in Savitsky et al. 2025.
These mutations are neomorphic; they not only damage the protein’s normal function but also give it new, harmful features. The altered Gαo proteins bind too tightly to their receptors and to Ric8A and Ric8B, helper molecules that assist many different G proteins, and thus blocking entire signaling pathways. These insights provide a key piece of the puzzle and corroborate the findings of their recent study published in the Journal of Clinical Investigation.
The severe symptoms caused by these neomorphic mutations confirm that tiny molecular changes can have severe physiological consequences.
What’s next?
By tracing the effects of altered Gαo proteins, researchers have uncovered how tiny cellular changes can result in extreme disturbances to brain activity and movement. To better understand how these mutations act, they aim now to study their impact on gene expression and protein production by reprogramming induced pluripotent stem (iPS) cells to mimic physiological conditions.
