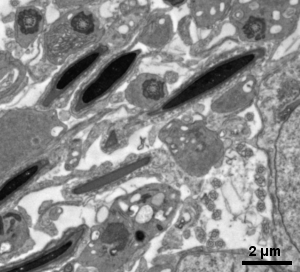
Resin embedded ultrathin section TEM
Principles:
The cellular ultrastructure of biological samples (cells or tissues) imaged with transmission electron microscope (TEM) is accessible through ultrathin-sections (50 - 80 nm) from the specimen embedded in epoxy resin cured block.
Sample preparation is lengthy and sophisticated procedure started from conventionally chemically fixed samples in mixture of glutaraldehyde and paraformaldehyde follwed by contrasting membranes, phospholipids and nucleic acids with heavy metal elements (e.g. osmium tetroxide, tannic acid, uranyl acetate, lead aspartate, ...), dehydration with grade series of ethanol (or acetone, propylen oxide) and gradual infiltration with epoxy resin and final embedding in pure resin and polymerization at 60°C to cure and harden the sample block (e.g in flat mold, gelatine capsule, PCR tube,...)
Finally, the cure resin block is mounted to the ultramicrotome, cutting face is trimmed to the recquired shape and size and ultra-thin sections cut with diamond knife are floating on the water surface of the knife's boat. Sections are collected on the EM grids, dried and optionally post-stained with uranyl acetate and lead citrate prior TEM examination and imaging.
Application:
Method of choice to study the intra-cellular ultrastructure of cells, tissues, organoids, ...
Method:
- Small piece (1 - 2 mm3) of tissue or cell pellet or attached cells on coverslip are chemically fixed with 2.5% glutaraldehyde and 2% paraformaldehyde mixture in 0.1 M Na-cacodylate buffer for at least 1 h at room temperature (or overnight at 4 °C).
- Postfixation with 1% Os04 with 1.5% potassium ferrocyanide in 0.1 M Na-cacodylate buffer for 1 h.
- Postfixation with 1% Os04 alone in 0.1 M Na-cacodylate buffer for 1h.
- Wash with double distilled water for 2 × 5 min.
- En block staining with aqueous 1% uranyl acetate for 1 h (or over night at 4°C).
- Wash with double distilled water for 2 × 5 min.
- Dehydration with graded ethanol series (2 × 50%, 70%, 90%, 95% and 2 × absolute ethanol) for 10 min each wash.
- Gradual epoxy resin infiltration diluted with ethanol at 1:2, 1:1 and 2:1 for 30 min each.
- Sample infiltration in pure resin 2 × 30 min and with fresh pure resin for additional 2-4 hrs.
- Sample embedding into fresh resin filled flat molds or gelatine capsules or small PCR tube.
- Resin polymerization at 60°C oven for 24-48 hrs.
Notes:
- Facility only accepts FIXED SAMPLE: users responsibility to properly fix the sample(s) after disscussed and advised with PFMU staff
- Fixation mixture should be buffered with buffer optimized for your sample. We prefer inert sodium cacodylate buffer but if this no compatible with your sample, use the original buffer which after fixation step can be exchanged to the cacodylate one by extensive multiple washes (e.g. 5 - 6 times for 5 min each wash)
- To minimaze material loss from cell pellet after osmium post-fixation the pellet is infiltrated into 1-3 % aqueous agarose solution, spin down, solidified on ice and cut to small (1 - 2 mm3) pieces
- Dehydration incubation step of tissue sample can be prolonged up to 30 min for each wash
- Epoxy resin commonle used is EPON or Durcupan
Gallery of different samples prepared for TEM

Mouse skeletal muscle |

Mouse skeletal musle |
|

Next soon ... |
||