How the most severe GNAO1 mutations disrupt brain signaling
Mutations in the GNAO1 gene can lead to severe neurological disorders, leading to treatment-resistant epilepsy, developmental delay and movement disorders in children. Zinc supplementation has emerged as a promising therapeutic option, helping to reduce symptoms. However, it does not completely repair the defects.
GNAO1 mutations disrupt signaling…
In a recent study published in the FASEB journal, researchers from the laboratory of Prof. Vladimir Katanev investigated the most frequent and severe GNAO1 mutations found in patients. The GNAO1 gene encodes Gαo, a key signaling protein that enables brain cells to respond to chemical messages through cell-surface GPCR receptors. Previous studies had proposed conflicting explanations for how mutant proteins disrupt this process, pointing to two distinct and incompatible mechanisms: either a strong and abnormal binding with its partner proteins Gβ and Gγ or with the GPCR receptors.
… by blocking GPCR receptors
To disentangle this, the team developed a new fluorescent imaging assay that allows direct visualization of receptor– Gαo protein interaction in living cells after chemical stimulation, fluorescence arising only when and where the two non-fluorescence tags (left panel, in green) are binding. Using this approach, they found that, in contrast to healthy cells where the Gαo protein allows receptor internalization from the cell surface to transmit the message (middle panel, white dots inside the cells), the most severe mutations cause the Gαo protein to remain abnormally attached to cell surface GPCR receptors (right panel).
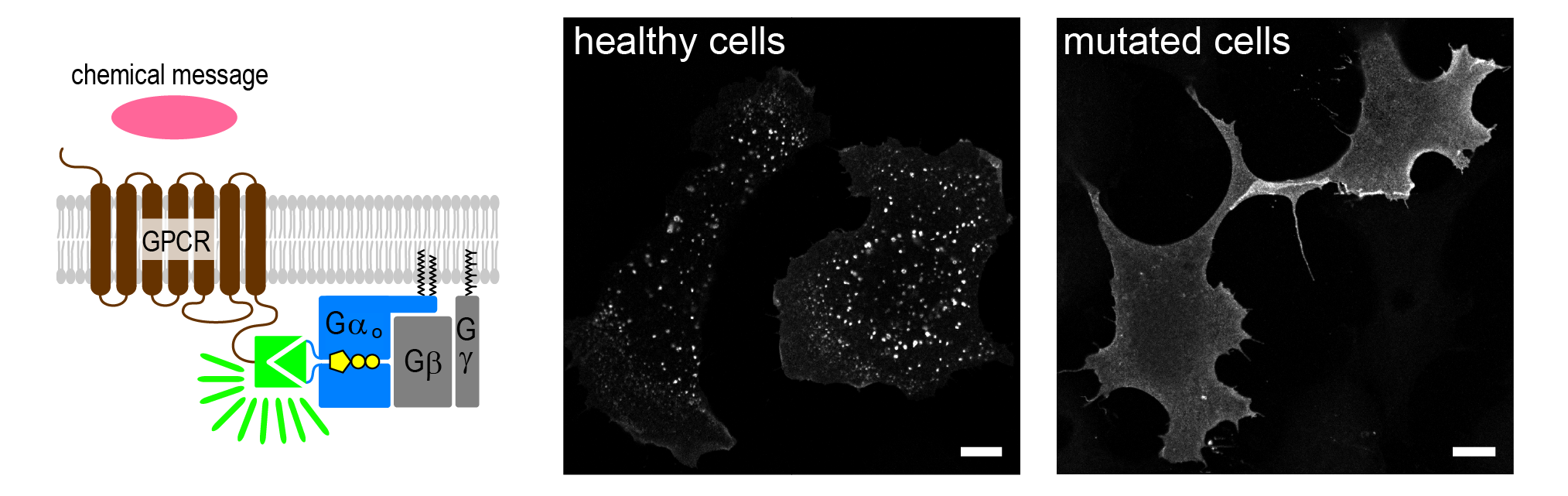
A new fluorescent imaging assay that allows direct visualization of receptor– Gαo protein interaction in living cells after chemical stimulation. In contrast to healthy cells where the Gαo protein allows receptor detachment from cell surface to transmit the message (middle panel, white dots inside the cells), the most severe mutations cause the Gαo protein to remain abnormally attached to cell surface GPCR receptors (right panel). Adapted from Figure 1 in Larasati et al 2026.
Our new fluorescent assay allows us to watch Gαo-receptor interactions in living cells.
What’s next?
By defining a shared molecular mechanism for severe GNAO1 diseases, this work opens new avenues for developing targeted strategies to correct disrupted brain signaling. The research team is now screening thousands of approved drugs to determine if some of them are able to uncouple Gαo mutants from GPCR receptors and restore a proper signaling.
